HAEM5:Plasmablastic lymphoma: Difference between revisions
| [unchecked revision] | [unchecked revision] |
Bailey.Glen (talk | contribs) No edit summary |
Bailey.Glen (talk | contribs) No edit summary |
||
| Line 7: | Line 7: | ||
}}</blockquote> | }}</blockquote> | ||
<span style="color:#0070C0">(General Instructions – The | <span style="color:#0070C0">(General Instructions – The focus of these pages is the clinically significant genetic alterations in each disease type. This is based on up-to-date knowledge from multiple resources such as PubMed and the WHO classification books. The CCGA is meant to be a supplemental resource to the WHO classification books; the CCGA captures in a continually updated wiki-stye manner the current genetics/genomics knowledge of each disease, which evolves more rapidly than books can be revised and published. If the same disease is described in multiple WHO classification books, the genetics-related information for that disease will be consolidated into a single main page that has this template (other pages would only contain a link to this main page). Use [https://www.genenames.org/ <u>HUGO-approved gene names and symbols</u>] (italicized when appropriate), [https://varnomen.hgvs.org/ <u>HGVS-based nomenclature for variants</u>], as well as generic names of drugs and testing platforms or assays if applicable. Please complete tables whenever possible and do not delete them (add N/A if not applicable in the table and delete the examples); to add (or move) a row or column in a table, click nearby within the table and select the > symbol that appears. Please do not delete or alter the section headings. The use of bullet points alongside short blocks of text rather than only large paragraphs is encouraged. Additional instructions below in italicized blue text should not be included in the final page content. Please also see </span><u>[[Author_Instructions]]</u><span style="color:#0070C0"> and [[Frequently Asked Questions (FAQs)|<u>FAQs</u>]] as well as contact your [[Leadership|<u>Associate Editor</u>]] or [mailto:CCGA@cancergenomics.org <u>Technical Support</u>].)</span> | ||
==Primary Author(s)*== | ==Primary Author(s)*== | ||
| Line 124: | Line 124: | ||
---- | ---- | ||
</blockquote> | </blockquote> | ||
== | ==WHO Essential and Desirable Genetic Diagnostic Criteria== | ||
<span style="color:#0070C0">(''Instructions: The table will have the diagnostic criteria from the WHO book <u>autocompleted</u>; remove any <u>non</u>-genetics related criteria. If applicable, add text about other classification'' ''systems that define this entity and specify how the genetics-related criteria differ.'')</span> | |||
{| class="wikitable" | |||
|+ | |||
|WHO Essential Criteria (Genetics)* | |||
| | |||
|- | |||
|WHO Desirable Criteria (Genetics)* | |||
| | |||
|- | |||
|Other Classification | |||
| | |||
|} | |||
<nowiki>*</nowiki>Note: These are only the genetic/genomic criteria. Additional diagnostic criteria can be found in the [https://tumourclassification.iarc.who.int/home <u>WHO Classification of Tumours</u>]. | |||
==Related Terminology== | |||
<span style="color:#0070C0">(''Instructions: The table will have the related terminology from the WHO <u>autocompleted</u>.)''</span> | |||
{| class="wikitable" | |||
|+ | |||
|Acceptable | |||
| | |||
|- | |||
|Not Recommended | |||
| | |||
|} | |||
==Gene Rearrangements== | |||
Put your text here and fill in the table <span style="color:#0070C0">(''Instructions: Details on clinical significance such as prognosis and other important information can be provided in the notes section. Please include references throughout the table. Do not delete the table.'')</span> | |||
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
|- | |- | ||
! | !Driver Gene!!Fusion(s) and Common Partner Genes!!Molecular Pathogenesis!!Typical Chromosomal Alteration(s) | ||
!Diagnostic Significance | !Prevalence -Common >20%, Recurrent 5-20% or Rare <5% (Disease) | ||
! | !Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | ||
! | !Established Clinical Significance Per Guidelines - Yes or No (Source) | ||
!Clinical Relevance Details/Other Notes | |||
|- | |||
|<span class="blue-text">EXAMPLE:</span> ''ABL1''||<span class="blue-text">EXAMPLE:</span> ''BCR::ABL1''||<span class="blue-text">EXAMPLE:</span> The pathogenic derivative is the der(22) resulting in fusion of 5’ BCR and 3’ABL1.||<span class="blue-text">EXAMPLE:</span> t(9;22)(q34;q11.2) | |||
|<span class="blue-text">EXAMPLE:</span> Common (CML) | |||
|<span class="blue-text">EXAMPLE:</span> D, P, T | |||
|<span class="blue-text">EXAMPLE:</span> Yes (WHO, NCCN) | |||
|<span class="blue-text">EXAMPLE:</span> | |||
The t(9;22) is diagnostic of CML in the appropriate morphology and clinical context (add reference). This fusion is responsive to targeted therapy such as Imatinib (Gleevec) (add reference). BCR::ABL1 is generally favorable in CML (add reference). | |||
|- | |- | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> ''CIC'' | ||
<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> ''CIC::DUX4'' | ||
| | |<span class="blue-text">EXAMPLE:</span> Typically, the last exon of ''CIC'' is fused to ''DUX4''. The fusion breakpoint in ''CIC'' is usually intra-exonic and removes an inhibitory sequence, upregulating ''PEA3'' genes downstream of ''CIC'' including ''ETV1'', ''ETV4'', and ''ETV5''. | ||
|<span class="blue-text">EXAMPLE:</span> t(4;19)(q25;q13) | |||
| | |<span class="blue-text">EXAMPLE:</span> Common (CIC-rearranged sarcoma) | ||
|<span class="blue-text">EXAMPLE:</span> D | |||
| | |||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
''DUX4'' has many homologous genes; an alternate translocation in a minority of cases is t(10;19), but this is usually indistinguishable from t(4;19) by short-read sequencing (add references). | |||
|} | |- | ||
|<span class="blue-text">EXAMPLE:</span> ''ALK'' | |||
|<span class="blue-text">EXAMPLE:</span> ''ELM4::ALK'' | |||
Other fusion partners include ''KIF5B, NPM1, STRN, TFG, TPM3, CLTC, KLC1'' | |||
|<span class="blue-text">EXAMPLE:</span> Fusions result in constitutive activation of the ''ALK'' tyrosine kinase. The most common ''ALK'' fusion is ''EML4::ALK'', with breakpoints in intron 19 of ''ALK''. At the transcript level, a variable (5’) partner gene is fused to 3’ ''ALK'' at exon 20. Rarely, ''ALK'' fusions contain exon 19 due to breakpoints in intron 18. | |||
|<span class="blue-text">EXAMPLE:</span> N/A | |||
|<span class="blue-text">EXAMPLE:</span> Rare (Lung adenocarcinoma) | |||
|<span class="blue-text">EXAMPLE:</span> T | |||
| | |||
|<span class="blue-text">EXAMPLE:</span> | |||
Both balanced and unbalanced forms are observed by FISH (add references). | |||
|- | |||
|<span class="blue-text">EXAMPLE:</span> ''ABL1'' | |||
|<span class="blue-text">EXAMPLE:</span> N/A | |||
|<span class="blue-text">EXAMPLE:</span> Intragenic deletion of exons 2–7 in ''EGFR'' removes the ligand-binding domain, resulting in a constitutively active tyrosine kinase with downstream activation of multiple oncogenic pathways. | |||
|<span class="blue-text">EXAMPLE:</span> N/A | |||
|<span class="blue-text">EXAMPLE:</span> Recurrent (IDH-wildtype Glioblastoma) | |||
|<span class="blue-text">EXAMPLE:</span> D, P, T | |||
| | |||
| | |||
|- | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
|} | |||
<blockquote class='blockedit'>{{Box-round|title=v4:Chromosomal Rearrangements (Gene Fusions)|The content below was from the old template. Please incorporate above.}}</blockquote> | <blockquote class='blockedit'>{{Box-round|title=v4:Chromosomal Rearrangements (Gene Fusions)|The content below was from the old template. Please incorporate above.}}</blockquote> | ||
| Line 175: | Line 240: | ||
---- | ---- | ||
</blockquote> | </blockquote> | ||
==Individual Region Genomic Gain / Loss / LOH== | ==Individual Region Genomic Gain/Loss/LOH== | ||
Put your text here and fill in the table <span style="color:#0070C0">(''Instructions: Includes aberrations not involving gene rearrangements. Details on clinical significance such as prognosis and other important information can be provided in the notes section. Can refer to CGC workgroup tables as linked on the homepage if applicable. Please include references throughout the table. Do not delete the table.'') </span> | |||
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
|- | |- | ||
!Chr #!!Gain | !Chr #!!'''Gain, Loss, Amp, LOH'''!!'''Minimal Region Cytoband and/or Genomic Coordinates [Genome Build; Size]'''!!'''Relevant Gene(s)''' | ||
!Diagnostic | !'''Diagnostic, Prognostic, and Therapeutic Significance - D, P, T''' | ||
!'''Established Clinical Significance Per Guidelines - Yes or No (Source)''' | |||
! | !'''Clinical Relevance Details/Other Notes''' | ||
!Notes | |||
|- | |- | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
7 | 7 | ||
|<span class="blue-text">EXAMPLE:</span> Loss | |<span class="blue-text">EXAMPLE:</span> Loss | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
chr7 | |||
chr7 | |||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
Unknown | |||
|<span class="blue-text">EXAMPLE:</span> D, P | |||
|<span class="blue-text">EXAMPLE:</span> No | |||
| | |||
|No | |||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
Presence of monosomy 7 (or 7q deletion) is sufficient for a diagnosis of AML with MDS-related changes when there is ≥20% blasts and no prior therapy (add reference). Monosomy 7/7q deletion is associated with a poor prognosis in AML (add references). | |||
Presence of monosomy 7 (or 7q deletion) is sufficient for a diagnosis of AML with MDS-related changes when there is ≥20% blasts and no prior therapy (add reference). Monosomy 7/7q deletion is associated with a poor prognosis in AML (add | |||
|- | |- | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
8 | 8 | ||
|<span class="blue-text">EXAMPLE:</span> Gain | |<span class="blue-text">EXAMPLE:</span> Gain | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
chr8 | |||
|<span class="blue-text">EXAMPLE:</span> | |||
Unknown | |||
|<span class="blue-text">EXAMPLE:</span> D, P | |||
| | |||
|<span class="blue-text">EXAMPLE:</span> | |||
Common recurrent secondary finding for t(8;21) (add references). | |||
|- | |||
|<span class="blue-text">EXAMPLE:</span> | |||
17 | |||
|<span class="blue-text">EXAMPLE:</span> Amp | |||
|<span class="blue-text">EXAMPLE:</span> | |||
17q12; chr17:39,700,064-39,728,658 [hg38; 28.6 kb] | |||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
''ERBB2'' | |||
|<span class="blue-text">EXAMPLE:</span> D, P, T | |||
| | |||
| | |||
| | |||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
Amplification of ''ERBB2'' is associated with HER2 overexpression in HER2 positive breast cancer (add references). Add criteria for how amplification is defined. | |||
|- | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
|} | |} | ||
| Line 230: | Line 304: | ||
---- | ---- | ||
</blockquote> | </blockquote> | ||
==Characteristic Chromosomal Patterns== | ==Characteristic Chromosomal or Other Global Mutational Patterns== | ||
Put your text here and fill in the table <span style="color:#0070C0">(I''nstructions: Included in this category are alterations such as hyperdiploid; gain of odd number chromosomes including typically chromosome 1, 3, 5, 7, 11, and 17; co-deletion of 1p and 19q; complex karyotypes without characteristic genetic findings; chromothripsis; microsatellite instability; homologous recombination deficiency; mutational signature pattern; etc. Details on clinical significance such as prognosis and other important information can be provided in the notes section. Please include references throughout the table. Do not delete the table.'')</span> | |||
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
|- | |- | ||
!Chromosomal Pattern | !Chromosomal Pattern | ||
! | !Molecular Pathogenesis | ||
!Prognostic Significance | !'''Prevalence -''' | ||
! | '''Common >20%, Recurrent 5-20% or Rare <5% (Disease)''' | ||
!Notes | !'''Diagnostic, Prognostic, and Therapeutic Significance - D, P, T''' | ||
!'''Established Clinical Significance Per Guidelines - Yes or No (Source)''' | |||
!'''Clinical Relevance Details/Other Notes''' | |||
|- | |- | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
Co-deletion of 1p and 18q | Co-deletion of 1p and 18q | ||
| | |<span class="blue-text">EXAMPLE:</span> See chromosomal rearrangements table as this pattern is due to an unbalanced derivative translocation associated with oligodendroglioma (add reference). | ||
| | |<span class="blue-text">EXAMPLE:</span> Common (Oligodendroglioma) | ||
| | |<span class="blue-text">EXAMPLE:</span> D, P | ||
| | |||
| | |||
|- | |||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
Microsatellite instability - hypermutated | |||
| | |||
|<span class="blue-text">EXAMPLE:</span> Common (Endometrial carcinoma) | |||
|<span class="blue-text">EXAMPLE:</span> P, T | |||
| | |||
| | |||
|- | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
|} | |} | ||
| Line 261: | Line 350: | ||
---- | ---- | ||
</blockquote> | </blockquote> | ||
==Gene Mutations (SNV / INDEL)== | ==Gene Mutations (SNV/INDEL)== | ||
Put your text here and fill in the table <span style="color:#0070C0">(''Instructions: This table is not meant to be an exhaustive list; please include only genes/alterations that are recurrent or common as well either disease defining and/or clinically significant. If a gene has multiple mechanisms depending on the type or site of the alteration, add multiple entries in the table. For clinical significance, denote associations with FDA-approved therapy (not an extensive list of applicable drugs) and NCCN or other national guidelines if applicable; Can also refer to CGC workgroup tables as linked on the homepage if applicable as well as any high impact papers or reviews of gene mutations in this entity. Details on clinical significance such as prognosis and other important information such as concomitant and mutually exclusive mutations can be provided in the notes section. Please include references throughout the table. Do not delete the table.'') </span> | |||
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
|- | |- | ||
!Gene | !Gene!!'''Genetic Alteration'''!!'''Tumor Suppressor Gene, Oncogene, Other'''!!'''Prevalence -''' | ||
!''' | '''Common >20%, Recurrent 5-20% or Rare <5% (Disease)''' | ||
! | !'''Diagnostic, Prognostic, and Therapeutic Significance - D, P, T ''' | ||
!'''Established Clinical Significance Per Guidelines - Yes or No (Source)''' | |||
!'''Clinical Relevance Details/Other Notes''' | |||
|- | |- | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span>''EGFR'' | ||
<span class="blue-text">EXAMPLE:</span> | <br /> | ||
|<span class="blue-text">EXAMPLE:</span> Exon 18-21 activating mutations | |||
|<span class="blue-text">EXAMPLE:</span> Oncogene | |||
|<span class="blue-text">EXAMPLE:</span> Common (lung cancer) | |||
<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> T | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> Yes (NCCN) | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> Exons 18, 19, and 21 mutations are targetable for therapy. Exon 20 T790M variants cause resistance to first generation TKI therapy and are targetable by second and third generation TKIs (add references). | ||
|- | |||
<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> ''TP53''; Variable LOF mutations | ||
|<span class="blue-text">EXAMPLE:</span> | <br /> | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> Variable LOF mutations | ||
|<span class="blue-text">EXAMPLE:</span> Tumor Supressor Gene | |||
|<span class="blue-text">EXAMPLE:</span> Common (breast cancer) | |||
|<span class="blue-text">EXAMPLE:</span> P | |||
| | |||
|<span class="blue-text">EXAMPLE:</span> >90% are somatic; rare germline alterations associated with Li-Fraumeni syndrome (add reference). Denotes a poor prognosis in breast cancer. | |||
|- | |||
|<span class="blue-text">EXAMPLE:</span> ''BRAF''; Activating mutations | |||
|<span class="blue-text">EXAMPLE:</span> Activating mutations | |||
|<span class="blue-text">EXAMPLE:</span> Oncogene | |||
|<span class="blue-text">EXAMPLE:</span> Common (melanoma) | |||
|<span class="blue-text">EXAMPLE:</span> T | |||
| | |||
| | |||
|- | |||
| | |||
| | |||
| | |||
| | |||
| | | | ||
| | | | ||
| | | | ||
|}Note: A more extensive list of mutations can be found in [https://www.cbioportal.org/ <u>cBioportal</u>], [https://cancer.sanger.ac.uk/cosmic <u>COSMIC</u>], and/or other databases. When applicable, gene-specific pages within the CCGA site directly link to pertinent external content. | |||
|} | |||
Note: A more extensive list of mutations can be found in | |||
<blockquote class='blockedit'>{{Box-round|title=v4:Gene Mutations (SNV/INDEL)|The content below was from the old template. Please incorporate above.}}</blockquote> | <blockquote class='blockedit'>{{Box-round|title=v4:Gene Mutations (SNV/INDEL)|The content below was from the old template. Please incorporate above.}}</blockquote> | ||
| Line 313: | Line 416: | ||
==Genes and Main Pathways Involved== | ==Genes and Main Pathways Involved== | ||
Put your text here and fill in the table <span style="color:#0070C0">(''Instructions: | |||
Put your text here and fill in the table <span style="color:#0070C0">(''Instructions: Please include references throughout the table. Do not delete the table.)''</span> | |||
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
|- | |- | ||
!Gene; Genetic Alteration!!Pathway!!Pathophysiologic Outcome | !Gene; Genetic Alteration!!Pathway!!Pathophysiologic Outcome | ||
|- | |- | ||
|<span class="blue-text">EXAMPLE:</span> BRAF and MAP2K1; Activating mutations | |<span class="blue-text">EXAMPLE:</span> ''BRAF'' and ''MAP2K1''; Activating mutations | ||
|<span class="blue-text">EXAMPLE:</span> MAPK signaling | |<span class="blue-text">EXAMPLE:</span> MAPK signaling | ||
|<span class="blue-text">EXAMPLE:</span> Increased cell growth and proliferation | |<span class="blue-text">EXAMPLE:</span> Increased cell growth and proliferation | ||
|- | |- | ||
|<span class="blue-text">EXAMPLE:</span> CDKN2A; Inactivating mutations | |<span class="blue-text">EXAMPLE:</span> ''CDKN2A''; Inactivating mutations | ||
|<span class="blue-text">EXAMPLE:</span> Cell cycle regulation | |<span class="blue-text">EXAMPLE:</span> Cell cycle regulation | ||
|<span class="blue-text">EXAMPLE:</span> Unregulated cell division | |<span class="blue-text">EXAMPLE:</span> Unregulated cell division | ||
|- | |- | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> ''KMT2C'' and ''ARID1A''; Inactivating mutations | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> Histone modification, chromatin remodeling | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> Abnormal gene expression program | ||
|- | |||
| | |||
| | |||
| | |||
|} | |} | ||
| Line 364: | Line 472: | ||
==References== | ==References== | ||
(use the "Cite" icon at the top of the page) <span style="color:#0070C0">(''Instructions: Add each reference into the text above by clicking | (use the "Cite" icon at the top of the page) <span style="color:#0070C0">(''Instructions: Add each reference into the text above by clicking where you want to insert the reference, selecting the “Cite” icon at the top of the wiki page, and using the “Automatic” tab option to search by PMID to select the reference to insert. If a PMID is not available, such as for a book, please use the “Cite” icon, select “Manual” and then “Basic Form”, and include the entire reference. To insert the same reference again later in the page, select the “Cite” icon and “Re-use” to find the reference; DO NOT insert the same reference twice using the “Automatic” tab as it will be treated as two separate references. The reference list in this section will be automatically generated and sorted''</span><span style="color:#0070C0">''.''</span><span style="color:#0070C0">)</span> <references /> | ||
''' | ''' | ||
==Notes== | ==Notes== | ||
<nowiki>*</nowiki>Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA | <nowiki>*</nowiki>Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the [[Leadership|''<u>Associate Editor</u>'']] or other CCGA representative. When pages have a major update, the new author will be acknowledged at the beginning of the page, and those who contributed previously will be acknowledged below as a prior author. | ||
Prior Author(s): | |||
<nowiki>*</nowiki>''Citation of this Page'': “Plasmablastic lymphoma”. Compendium of Cancer Genome Aberrations (CCGA), Cancer Genomics Consortium (CGC), updated {{REVISIONMONTH}}/{{REVISIONDAY}}/{{REVISIONYEAR}}, <nowiki>https://ccga.io/index.php/HAEM5:Plasmablastic_lymphoma</nowiki>. | <nowiki>*</nowiki>''Citation of this Page'': “Plasmablastic lymphoma”. Compendium of Cancer Genome Aberrations (CCGA), Cancer Genomics Consortium (CGC), updated {{REVISIONMONTH}}/{{REVISIONDAY}}/{{REVISIONYEAR}}, <nowiki>https://ccga.io/index.php/HAEM5:Plasmablastic_lymphoma</nowiki>. | ||
[[Category:HAEM5]][[Category:DISEASE]][[Category:Diseases P]] | [[Category:HAEM5]][[Category:DISEASE]][[Category:Diseases P]] | ||
Revision as of 13:57, 10 February 2025
Haematolymphoid Tumours (WHO Classification, 5th ed.)
| This page is under construction |
editContent Update To WHO 5th Edition Classification Is In Process; Content Below is Based on WHO 4th Edition ClassificationThis page was converted to the new template on 2023-12-07. The original page can be found at HAEM4:Plasmablastic Lymphoma.
(General Instructions – The focus of these pages is the clinically significant genetic alterations in each disease type. This is based on up-to-date knowledge from multiple resources such as PubMed and the WHO classification books. The CCGA is meant to be a supplemental resource to the WHO classification books; the CCGA captures in a continually updated wiki-stye manner the current genetics/genomics knowledge of each disease, which evolves more rapidly than books can be revised and published. If the same disease is described in multiple WHO classification books, the genetics-related information for that disease will be consolidated into a single main page that has this template (other pages would only contain a link to this main page). Use HUGO-approved gene names and symbols (italicized when appropriate), HGVS-based nomenclature for variants, as well as generic names of drugs and testing platforms or assays if applicable. Please complete tables whenever possible and do not delete them (add N/A if not applicable in the table and delete the examples); to add (or move) a row or column in a table, click nearby within the table and select the > symbol that appears. Please do not delete or alter the section headings. The use of bullet points alongside short blocks of text rather than only large paragraphs is encouraged. Additional instructions below in italicized blue text should not be included in the final page content. Please also see Author_Instructions and FAQs as well as contact your Associate Editor or Technical Support.)
Primary Author(s)*
Mark Evans, MD (University of California, Irvine)
Fabiola Quintero-Rivera, MD (University of California, Irvine)
WHO Classification of Disease
| Structure | Disease |
|---|---|
| Book | Haematolymphoid Tumours (5th ed.) |
| Category | B-cell lymphoid proliferations and lymphomas |
| Family | Mature B-cell neoplasms |
| Type | Large B-cell lymphomas |
| Subtype(s) | Plasmablastic lymphoma |
Definition / Description of Disease
In 1997, Delecluse et al. described a series of large B-cell lymphomas occurring within the jaw and oral cavities of HIV-positive individuals[1]. The cells were blastic in appearance and did not express CD20, but did demonstrate plasmacytic antigens. Plasmablastic lymphoma (PBL) is recognized by the World Health Organization (WHO) as an aggressive proliferation of large B cells with immunoblastic or plasmablastic morphology and plasmacytic phenotype[2]. This entity is distinguished from other large B-cell lymphomas with similar immunoprofiles, such as ALK-positive large B-cell lymphoma and HHV-8-associated lymphoproliferative disorders.
Synonyms / Terminology
Monomorphic plasmablastic lymphoma; plasmablastic lymphoma with plasmacytic differentiation
Epidemiology / Prevalence
- Plasmablastic lymphoma typically occurs in adults with human immunodeficiency virus (HIV) infection (approximately 73% of cases)[3].
- It is also seen in the setting of iatrogenic immunosuppression (autoimmune disease or post-transplant)[4].
- PBL has been observed in older immunocompetent adults and in children, typically with HIV or immunodeficiency[5][6].
Clinical Features
Put your text here and fill in the table (Instruction: Can include references in the table. Do not delete table.)
| Signs and Symptoms | EXAMPLE: Asymptomatic (incidental finding on complete blood counts)
EXAMPLE: B-symptoms (weight loss, fever, night sweats) EXAMPLE: Fatigue EXAMPLE: Lymphadenopathy (uncommon) |
| Laboratory Findings | EXAMPLE: Cytopenias
EXAMPLE: Lymphocytosis (low level) |
editv4:Clinical FeaturesThe content below was from the old template. Please incorporate above.
An extranodal mass is the most typical presentation, and nodal disease is more common in post-transplant PBL[3]. Paraproteins may be detected in some cases[7]. Greater than 50% of cases associated with some form of immunodeficiency present with stage III/IV disease with bone marrow involvement[3][5].
End of V4 Section
Sites of Involvement
Typically head and neck regions, particularly the oral cavity. Other less commonly involved sites include the gastrointestinal tract, skin, soft tissue, lung, bone, and rarely lymph nodes[8].
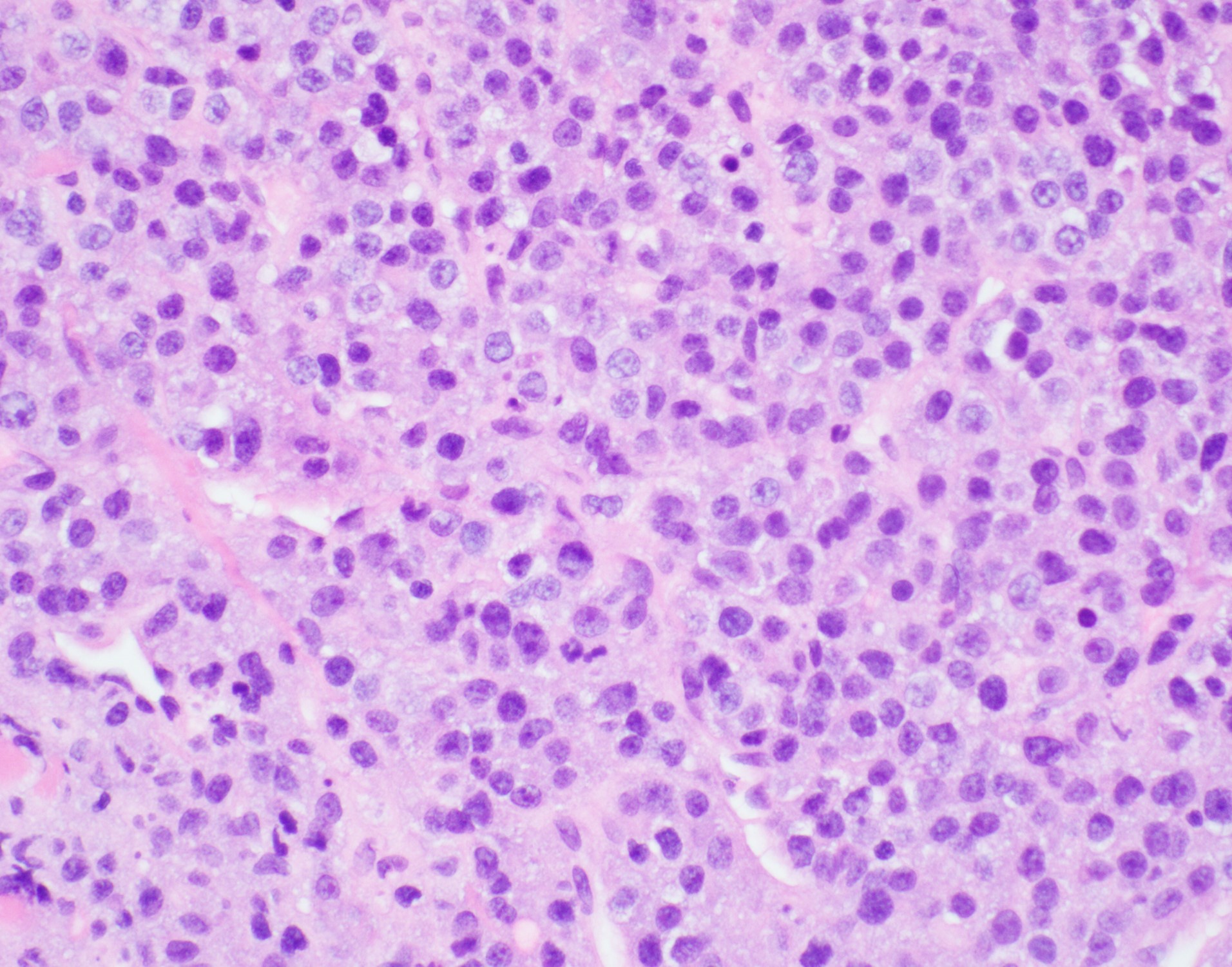
Morphologic Features
Two histologic variants have been described:
- The monomorphic variant features large immunoblast-like cells with fine nuclear chromatin, prominent nucleoli, and little or no plasmacytic differentiation; a starry sky pattern is common.
- The variant with plasmacytic differentiation is composed of cells with course nuclear chromatin, basophilic cytoplasm, eccentric nuclei, and paranuclear hof.
- Some cases demonstrate features of both variants[2].
Immunophenotype
Put your text here and fill in the table (Instruction: Can include references in the table. Do not delete table.)
| Finding | Marker |
|---|---|
| Positive (universal) | EXAMPLE: CD1 |
| Positive (subset) | EXAMPLE: CD2 |
| Negative (universal) | EXAMPLE: CD3 |
| Negative (subset) | EXAMPLE: CD4 |
editv4:ImmunophenotypeThe content below was from the old template. Please incorporate above.
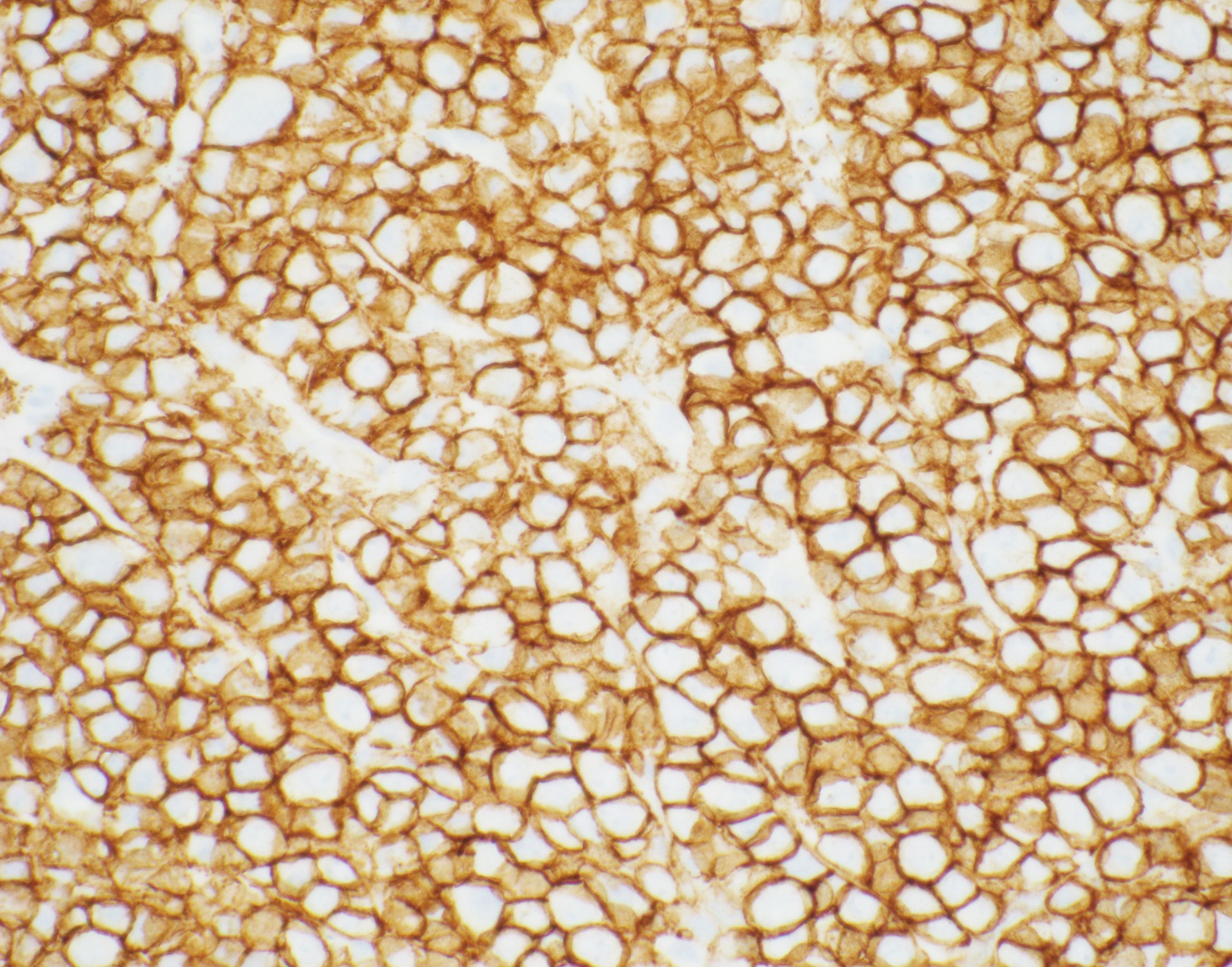
The cells express plasmacytic antigens (CD138, VS38c, IRF4/MUM1, BLIMP1, XBP1, and CD38). CD45, PAX-5, and CD20 are typically negative or weakly positive. Cytoplasmic IgG, as well as kappa and lambda light chains are common. CD79a is present in approximately 40% of cases, and CD56 in about 25%. The cells are typically positive for Epstein-Barr virus-encoded RNA (EBER). Ki-67 proliferation index is usually >90%[8][9].
End of V4 Section
WHO Essential and Desirable Genetic Diagnostic Criteria
(Instructions: The table will have the diagnostic criteria from the WHO book autocompleted; remove any non-genetics related criteria. If applicable, add text about other classification systems that define this entity and specify how the genetics-related criteria differ.)
| WHO Essential Criteria (Genetics)* | |
| WHO Desirable Criteria (Genetics)* | |
| Other Classification |
*Note: These are only the genetic/genomic criteria. Additional diagnostic criteria can be found in the WHO Classification of Tumours.
Related Terminology
(Instructions: The table will have the related terminology from the WHO autocompleted.)
| Acceptable | |
| Not Recommended |
Gene Rearrangements
Put your text here and fill in the table (Instructions: Details on clinical significance such as prognosis and other important information can be provided in the notes section. Please include references throughout the table. Do not delete the table.)
| Driver Gene | Fusion(s) and Common Partner Genes | Molecular Pathogenesis | Typical Chromosomal Alteration(s) | Prevalence -Common >20%, Recurrent 5-20% or Rare <5% (Disease) | Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
|---|---|---|---|---|---|---|---|
| EXAMPLE: ABL1 | EXAMPLE: BCR::ABL1 | EXAMPLE: The pathogenic derivative is the der(22) resulting in fusion of 5’ BCR and 3’ABL1. | EXAMPLE: t(9;22)(q34;q11.2) | EXAMPLE: Common (CML) | EXAMPLE: D, P, T | EXAMPLE: Yes (WHO, NCCN) | EXAMPLE:
The t(9;22) is diagnostic of CML in the appropriate morphology and clinical context (add reference). This fusion is responsive to targeted therapy such as Imatinib (Gleevec) (add reference). BCR::ABL1 is generally favorable in CML (add reference). |
| EXAMPLE: CIC | EXAMPLE: CIC::DUX4 | EXAMPLE: Typically, the last exon of CIC is fused to DUX4. The fusion breakpoint in CIC is usually intra-exonic and removes an inhibitory sequence, upregulating PEA3 genes downstream of CIC including ETV1, ETV4, and ETV5. | EXAMPLE: t(4;19)(q25;q13) | EXAMPLE: Common (CIC-rearranged sarcoma) | EXAMPLE: D | EXAMPLE:
DUX4 has many homologous genes; an alternate translocation in a minority of cases is t(10;19), but this is usually indistinguishable from t(4;19) by short-read sequencing (add references). | |
| EXAMPLE: ALK | EXAMPLE: ELM4::ALK
|
EXAMPLE: Fusions result in constitutive activation of the ALK tyrosine kinase. The most common ALK fusion is EML4::ALK, with breakpoints in intron 19 of ALK. At the transcript level, a variable (5’) partner gene is fused to 3’ ALK at exon 20. Rarely, ALK fusions contain exon 19 due to breakpoints in intron 18. | EXAMPLE: N/A | EXAMPLE: Rare (Lung adenocarcinoma) | EXAMPLE: T | EXAMPLE:
Both balanced and unbalanced forms are observed by FISH (add references). | |
| EXAMPLE: ABL1 | EXAMPLE: N/A | EXAMPLE: Intragenic deletion of exons 2–7 in EGFR removes the ligand-binding domain, resulting in a constitutively active tyrosine kinase with downstream activation of multiple oncogenic pathways. | EXAMPLE: N/A | EXAMPLE: Recurrent (IDH-wildtype Glioblastoma) | EXAMPLE: D, P, T | ||
editv4:Chromosomal Rearrangements (Gene Fusions)The content below was from the old template. Please incorporate above.
MYC (8q24) up-regulation occurs via translocations, frequently between MYC and immunoglobulin (IG) heavy chain [t(8;14)] and immunoglobulin light chain genes [t(2;8) or t(8;22)], which are also seen in Burkitt lymphoma. These translocations are more common in EBV-positive tumors (74%), and have been associated with poorer prognosis[10][11].
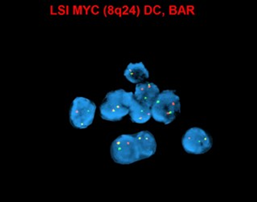
End of V4 Section
editv4:Clinical Significance (Diagnosis, Prognosis and Therapeutic Implications).Please incorporate this section into the relevant tables found in:
- Chromosomal Rearrangements (Gene Fusions)
- Individual Region Genomic Gain/Loss/LOH
- Characteristic Chromosomal Patterns
- Gene Mutations (SNV/INDEL)
- The prognosis of PBL is very poor, with three quarters of patients dying with a median survival of 6-11 months[3][12].
- There is currently no standard therapy for PBL. CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) have been generally considered inadequate, and the National Comprehensive Cancer Network (NCCN) favors Hyper-CVAD-MA (hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, and high-dose methotrexate and cytarabine), CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine), COMB (cyclophosphamide, Oncovin, methyl-CCNU, and bleomycin), and infusional EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin)[11][13][14].
- Patients with localized disease have a better prognosis, and these individuals can be managed by radiotherapy and doxorubicin-based chemotherapy with radiation therapy[15][16].
- Polychemotherapy is required for patients with disseminated disease; more than 50% achieve complete remissions (CRs), but approximately 70% die of progressive disease, with an event-free survival of 22 months, and an overall survival of 32 months[17].
End of V4 Section
Individual Region Genomic Gain/Loss/LOH
Put your text here and fill in the table (Instructions: Includes aberrations not involving gene rearrangements. Details on clinical significance such as prognosis and other important information can be provided in the notes section. Can refer to CGC workgroup tables as linked on the homepage if applicable. Please include references throughout the table. Do not delete the table.)
| Chr # | Gain, Loss, Amp, LOH | Minimal Region Cytoband and/or Genomic Coordinates [Genome Build; Size] | Relevant Gene(s) | Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
|---|---|---|---|---|---|---|
| EXAMPLE:
7 |
EXAMPLE: Loss | EXAMPLE:
chr7 |
EXAMPLE:
Unknown |
EXAMPLE: D, P | EXAMPLE: No | EXAMPLE:
Presence of monosomy 7 (or 7q deletion) is sufficient for a diagnosis of AML with MDS-related changes when there is ≥20% blasts and no prior therapy (add reference). Monosomy 7/7q deletion is associated with a poor prognosis in AML (add references). |
| EXAMPLE:
8 |
EXAMPLE: Gain | EXAMPLE:
chr8 |
EXAMPLE:
Unknown |
EXAMPLE: D, P | EXAMPLE:
Common recurrent secondary finding for t(8;21) (add references). | |
| EXAMPLE:
17 |
EXAMPLE: Amp | EXAMPLE:
17q12; chr17:39,700,064-39,728,658 [hg38; 28.6 kb] |
EXAMPLE:
ERBB2 |
EXAMPLE: D, P, T | EXAMPLE:
Amplification of ERBB2 is associated with HER2 overexpression in HER2 positive breast cancer (add references). Add criteria for how amplification is defined. | |
editv4:Genomic Gain/Loss/LOHThe content below was from the old template. Please incorporate above.
One study of 12 PBL cases showed recurrent gains of 1q31.1q44, 5p15.33p13.1, 7q11.2q11.23, 8q24.13q24.3, 11p and 11q terminal regions, 15q15q26.3, 19p13.3p13.12 and chromosomes 3, 7, 11, and 15, as well as losses of 1p36.33p35.1, 6q25.1q27, 8p23.3p22.14 and 18q21.32q23. Additionally, 54% of the cases had either deletion or copy neutral loss of heterozygosity (CN-LOH) involving the tumor suppressor gene CDKN2C at 1p32.3. Furthermore, recurrent copy number losses involving the immunoglobulin genes IGH and IGKV were documented[18].

End of V4 Section
Characteristic Chromosomal or Other Global Mutational Patterns
Put your text here and fill in the table (Instructions: Included in this category are alterations such as hyperdiploid; gain of odd number chromosomes including typically chromosome 1, 3, 5, 7, 11, and 17; co-deletion of 1p and 19q; complex karyotypes without characteristic genetic findings; chromothripsis; microsatellite instability; homologous recombination deficiency; mutational signature pattern; etc. Details on clinical significance such as prognosis and other important information can be provided in the notes section. Please include references throughout the table. Do not delete the table.)
| Chromosomal Pattern | Molecular Pathogenesis | Prevalence -
Common >20%, Recurrent 5-20% or Rare <5% (Disease) |
Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
|---|---|---|---|---|---|
| EXAMPLE:
Co-deletion of 1p and 18q |
EXAMPLE: See chromosomal rearrangements table as this pattern is due to an unbalanced derivative translocation associated with oligodendroglioma (add reference). | EXAMPLE: Common (Oligodendroglioma) | EXAMPLE: D, P | ||
| EXAMPLE:
Microsatellite instability - hypermutated |
EXAMPLE: Common (Endometrial carcinoma) | EXAMPLE: P, T | |||
editv4:Characteristic Chromosomal Aberrations / PatternsThe content below was from the old template. Please incorporate above.
In addition to the MYC/IG rearrangements, complex karyotypes are also frequently observed. PBL often demonstrates chromosomal changes seen in plasma cell myeloma, including gain of 1q, loss of 1p, deletions 13q and/or 17p, and gains of odd-numbered chromosomes, such as +3, +5, +7, +9, +11, and/or +15[7][19].
End of V4 Section
Gene Mutations (SNV/INDEL)
Put your text here and fill in the table (Instructions: This table is not meant to be an exhaustive list; please include only genes/alterations that are recurrent or common as well either disease defining and/or clinically significant. If a gene has multiple mechanisms depending on the type or site of the alteration, add multiple entries in the table. For clinical significance, denote associations with FDA-approved therapy (not an extensive list of applicable drugs) and NCCN or other national guidelines if applicable; Can also refer to CGC workgroup tables as linked on the homepage if applicable as well as any high impact papers or reviews of gene mutations in this entity. Details on clinical significance such as prognosis and other important information such as concomitant and mutually exclusive mutations can be provided in the notes section. Please include references throughout the table. Do not delete the table.)
| Gene | Genetic Alteration | Tumor Suppressor Gene, Oncogene, Other | Prevalence -
Common >20%, Recurrent 5-20% or Rare <5% (Disease) |
Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
|---|---|---|---|---|---|---|
| EXAMPLE:EGFR
|
EXAMPLE: Exon 18-21 activating mutations | EXAMPLE: Oncogene | EXAMPLE: Common (lung cancer) | EXAMPLE: T | EXAMPLE: Yes (NCCN) | EXAMPLE: Exons 18, 19, and 21 mutations are targetable for therapy. Exon 20 T790M variants cause resistance to first generation TKI therapy and are targetable by second and third generation TKIs (add references). |
| EXAMPLE: TP53; Variable LOF mutations
|
EXAMPLE: Variable LOF mutations | EXAMPLE: Tumor Supressor Gene | EXAMPLE: Common (breast cancer) | EXAMPLE: P | EXAMPLE: >90% are somatic; rare germline alterations associated with Li-Fraumeni syndrome (add reference). Denotes a poor prognosis in breast cancer. | |
| EXAMPLE: BRAF; Activating mutations | EXAMPLE: Activating mutations | EXAMPLE: Oncogene | EXAMPLE: Common (melanoma) | EXAMPLE: T | ||
Note: A more extensive list of mutations can be found in cBioportal, COSMIC, and/or other databases. When applicable, gene-specific pages within the CCGA site directly link to pertinent external content.
editv4:Gene Mutations (SNV/INDEL)The content below was from the old template. Please incorporate above.
- IGHV may be unmutated or demonstrate somatic hypermutation[20].
- Montes-Moreno et al. demonstrated somatic mutations in PRDM1(BLIMP1) in 50% of cases[21].
- The largest series of transplant-associated PBL analyzed by next-generation sequencing detected genetic aberrations of the RAS/MAPK, TP53, and NOTCH signaling pathways[22].
End of V4 Section
Epigenomic Alterations
Hypermethylation of p16 has been reported in a case of PBL[12].
Genes and Main Pathways Involved
Put your text here and fill in the table (Instructions: Please include references throughout the table. Do not delete the table.)
| Gene; Genetic Alteration | Pathway | Pathophysiologic Outcome |
|---|---|---|
| EXAMPLE: BRAF and MAP2K1; Activating mutations | EXAMPLE: MAPK signaling | EXAMPLE: Increased cell growth and proliferation |
| EXAMPLE: CDKN2A; Inactivating mutations | EXAMPLE: Cell cycle regulation | EXAMPLE: Unregulated cell division |
| EXAMPLE: KMT2C and ARID1A; Inactivating mutations | EXAMPLE: Histone modification, chromatin remodeling | EXAMPLE: Abnormal gene expression program |
editv4:Genes and Main Pathways InvolvedThe content below was from the old template. Please incorporate above.
- MYC expression is suppressed by PRDM1 (BLIMP1) in terminally differentiated B cells; BLIMP1 encodes a transcriptional factor responsible for plasma cell differentiation[11].
- The MYC activation present in PBL (by gene amplification or translocation) results in cellular proliferation and survival upon overcoming PRDM1 (BLIMP1) repression.
End of V4 Section
Genetic Diagnostic Testing Methods
- Diagnosis is usually dependent on morphologic examination and immunohistochemistry demonstrating expression for plasmacytic antigens.
- Conventional cytogenetics has utility in detecting MYC rearrangement and amplification. Most common translocation involves MYC -IGH.
- Next-generation sequencing is helpful for identifying single nucleotide variants of PRDM1 and of genes in the of the RAS/MAPK, TP53, and NOTCH signaling pathways.
Familial Forms
Not applicable.
Additional Information
Put your text here
Links
HAEM4:Lymphomas Associated with HIV Infection
MYC in COSMIC (https://cancer.sanger.ac.uk/cell_lines/gene/analysis?ln=MYC)
References
(use the "Cite" icon at the top of the page) (Instructions: Add each reference into the text above by clicking where you want to insert the reference, selecting the “Cite” icon at the top of the wiki page, and using the “Automatic” tab option to search by PMID to select the reference to insert. If a PMID is not available, such as for a book, please use the “Cite” icon, select “Manual” and then “Basic Form”, and include the entire reference. To insert the same reference again later in the page, select the “Cite” icon and “Re-use” to find the reference; DO NOT insert the same reference twice using the “Automatic” tab as it will be treated as two separate references. The reference list in this section will be automatically generated and sorted.)
- ↑ Delecluse, H. J.; et al. (1997). "Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection". Blood. 89 (4): 1413–1420. ISSN 0006-4971. PMID 9028965.
- ↑ 2.0 2.1 Campo, E.; et al. (2016). Plasmablastic lymphoma. in World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th edition. Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; et al. Editors. IARC Press: Lyon, France. p 321-322.
- ↑ 3.0 3.1 3.2 3.3 Castillo, Jorge J.; et al. (2015). "The biology and treatment of plasmablastic lymphoma". Blood. 125 (15): 2323–2330. doi:10.1182/blood-2014-10-567479. ISSN 1528-0020. PMID 25636338.
- ↑ Borenstein, J.; et al. (2007). "Plasmablastic lymphomas may occur as post-transplant lymphoproliferative disorders". Histopathology. 51 (6): 774–777. doi:10.1111/j.1365-2559.2007.02870.x. ISSN 0309-0167. PMID 17944927.
- ↑ 5.0 5.1 Colomo, Lluís; et al. (2004). "Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities". The American Journal of Surgical Pathology. 28 (6): 736–747. doi:10.1097/01.pas.0000126781.87158.e3. ISSN 0147-5185. PMID 15166665.
- ↑ Liu, Fang; et al. (2012). "Plasmablastic lymphoma of the elderly: a clinicopathological comparison with age-related Epstein-Barr virus-associated B cell lymphoproliferative disorder". Histopathology. 61 (6): 1183–1197. doi:10.1111/j.1365-2559.2012.04339.x. ISSN 1365-2559. PMID 22958176.
- ↑ 7.0 7.1 Taddesse-Heath, Lekidelu; et al. (2010). "Plasmablastic lymphoma with MYC translocation: evidence for a common pathway in the generation of plasmablastic features". Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc. 23 (7): 991–999. doi:10.1038/modpathol.2010.72. ISSN 1530-0285. PMC 6344124. PMID 20348882.
- ↑ 8.0 8.1 Campo, E. (2017). Plasmablastic neoplasms other than plasma cell myeloma. in Hematopathology. 2nd edition. Jaffe, E.S.; Arber, D.A.; Campo, E.; et al. Editors. Elsevier: Philadelphia. p 465-472.
- ↑ Montes-Moreno, Santiago; et al. (2010). "Aggressive large B-cell lymphoma with plasma cell differentiation: immunohistochemical characterization of plasmablastic lymphoma and diffuse large B-cell lymphoma with partial plasmablastic phenotype". Haematologica. 95 (8): 1342–1349. doi:10.3324/haematol.2009.016113. ISSN 1592-8721. PMC 2913083. PMID 20418245.
- ↑ Bogusz, Agata M.; et al. (2009). "Plasmablastic lymphomas with MYC/IgH rearrangement: report of three cases and review of the literature". American Journal of Clinical Pathology. 132 (4): 597–605. doi:10.1309/AJCPFUR1BK0UODTS. ISSN 1943-7722. PMID 19762538.
- ↑ 11.0 11.1 11.2 Valera, Alexandra; et al. (2010). "IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas". The American Journal of Surgical Pathology. 34 (11): 1686–1694. doi:10.1097/PAS.0b013e3181f3e29f. ISSN 1532-0979. PMC 2982261. PMID 20962620.
- ↑ 12.0 12.1 Morscio, Julie; et al. (2014). "Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and posttransplant patients: single-center series of 25 cases and meta-analysis of 277 reported cases". The American Journal of Surgical Pathology. 38 (7): 875–886. doi:10.1097/PAS.0000000000000234. ISSN 1532-0979. PMID 24832164.
- ↑ Zelenetz, Andrew D.; et al. (2011). "Non-Hodgkin's lymphomas". Journal of the National Comprehensive Cancer Network: JNCCN. 9 (5): 484–560. doi:10.6004/jnccn.2011.0046. ISSN 1540-1413. PMID 21550968.
- ↑ Koizumi, Yusuke; et al. (2016). "Clinical and pathological aspects of human immunodeficiency virus-associated plasmablastic lymphoma: analysis of 24 cases". International Journal of Hematology. 104 (6): 669–681. doi:10.1007/s12185-016-2082-3. ISSN 1865-3774. PMID 27604616.
- ↑ Phipps, C.; et al. (2017). "Durable remission is achievable with localized treatment and reduction of immunosuppression in limited stage EBV-related plasmablastic lymphoma". Annals of Hematology. 96 (11): 1959–1960. doi:10.1007/s00277-017-3109-4. ISSN 1432-0584. PMID 28831541.
- ↑ Pinnix, Chelsea C.; et al. (2016). "Doxorubicin-Based Chemotherapy and Radiation Therapy Produces Favorable Outcomes in Limited-Stage Plasmablastic Lymphoma: A Single-Institution Review". Clinical Lymphoma, Myeloma & Leukemia. 16 (3): 122–128. doi:10.1016/j.clml.2015.12.008. ISSN 2152-2669. PMID 26795083.
- ↑ Tchernonog, E.; et al. (2017). "Clinical characteristics and prognostic factors of plasmablastic lymphoma patients: analysis of 135 patients from the LYSA group". Annals of Oncology: Official Journal of the European Society for Medical Oncology. 28 (4): 843–848. doi:10.1093/annonc/mdw684. ISSN 1569-8041. PMID 28031174.
- ↑ Ji, Jianling; et al. (2016-06). "Genomic Profiling of Plasmablastic Lymphoma Reveals Recurrent Copy Number Alterations and MYC Rearrangement as Common Genetic Abnormalities". Cancer Genetics. 209 (6): 290. doi:10.1016/j.cancergen.2016.04.021. ISSN 2210-7762. Check date values in:
|date=(help) - ↑ Meloni-Ehrig, Aurelia; et al. (2017). “Plasmablastic lymphoma (PBL)”. Atlas Genet Cytogenet Oncol Haematol. 21 (2): 67-70.
- ↑ Gaidano, Gianluca; et al. (2002). "Molecular histogenesis of plasmablastic lymphoma of the oral cavity". British Journal of Haematology. 119 (3): 622–628. doi:10.1046/j.1365-2141.2002.03872.x. ISSN 0007-1048. PMID 12437635.
- ↑ Montes-Moreno, Santiago; et al. (2017). "Plasmablastic lymphoma phenotype is determined by genetic alterations in MYC and PRDM1". Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc. 30 (1): 85–94. doi:10.1038/modpathol.2016.162. ISSN 1530-0285. PMID 27687004.
- ↑ Bhagat, Govind; et al. (2017). "Molecular Characterization of Post-Transplant Plasmablastic Lymphomas Implicates RAS, TP53, and NOTCH Mutations and MYC Deregulation in Disease Pathogenesis". Blood. 130 (Supplement 1): 4014–4014. doi:10.1182/blood.V130.Suppl_1.4014.4014. ISSN 0006-4971.
Notes
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the Associate Editor or other CCGA representative. When pages have a major update, the new author will be acknowledged at the beginning of the page, and those who contributed previously will be acknowledged below as a prior author.
Prior Author(s):
*Citation of this Page: “Plasmablastic lymphoma”. Compendium of Cancer Genome Aberrations (CCGA), Cancer Genomics Consortium (CGC), updated 02/10/2025, https://ccga.io/index.php/HAEM5:Plasmablastic_lymphoma.